American Family News spoke to Brad Miller, a former U.S. Army lieutenant colonel who previously served as an Army battalion commander. In October 2021, Miller was relieved of his battalion command within the 101st Airborne Division for refusing the COVID-19 shot under Defense Secretary Lloyd Austin’s unlawful direction.
At the time when a COVID-19 shot was mandated in the military, many service members were quick to cite “two very important laws” – to no avail, according to Miller. He says thousands rightfully argued that a so-called "vaccine," only available through Emergency Use Authorization (EUA), was unlawful; but sadly, their arguments were deliberately ignored.
 “When people fully understand these two laws, 10 USC §1107a and 21 USC § 360bbb,” Miller asserts, “they’ll realize the military has blatantly operated outside of the law.”
“When people fully understand these two laws, 10 USC §1107a and 21 USC § 360bbb,” Miller asserts, “they’ll realize the military has blatantly operated outside of the law.”
He points out that 10 USC §1107a codifies that there must be informed consent. “It says that a potential recipient must be informed of an option to accept or refuse a treatment,” he explains, adding that, “this can only be waived by the president.”
In addition, 21 USC § 360bbb stipulates that EUA products can only be used when there is no other approved drug available. “Codified in the law,” Miller notes, “your rights of refusal are protected, especially as it related to experimental products.”
Prior to its approval by the Food and Drug Administration (FDA) in October 2020, these same laws would have applied to the use of remdesivir to treat COVID-19 in service members and veterans. Between May 2020 and October 2020, the FDA had only issued an EUA status for the controversial drug.
Alternatives discounted
Interestingly, some people – Miller among them – would argue that hydroxychloroquine and ivermectin could have been viable alternatives to remdesivir.

“If other therapeutics like hydroxychloroquine or ivermectin had been available, then there would not have been a need for remdesivir as an EUA product,” Miller explains. “Technically, there would have been no need for emergency use because there were other products available ….”
He continues: “Some observers would say there was an intentional demonization campaign to delegitimize the use of some of these other therapeutics, deeming them inappropriate or inadequate as treatment protocols, therefore leaving the door open to pass an EUA for the COVID shot.
“If we accept the government's narrative that the COVID shots were produced in record time and then distributed equally as fast, then it’s at least possible that we could say that the government did not necessarily know what unintended adverse effects might be produced by these shots.”
However, this was not the case with remdesivir, says the former military officer. “Remdesivir had been used in other trial usages at various times with disastrous results,” Miller points out.
For example, as published by The New England Journal of Medicine in 2019, the use of remdesivir in a trial of four investigational therapies for Ebola in the Democratic Republic of Congo resulted in the highest mortality rate among participants, claiming the lives of 93 out of 175 (53.1%) of patients.
Miller argues that because of this readily available research, “The government knew how risky the use of remdesivir as a therapeutic was, but they propped it up as a legitimate treatment [for COVID-19] anyway.”
Tight-lipped about study results
The U.S. Army Medical Research and Development Command (USAMRC) sponsored a clinical trial to study the use of remdesivir to treat COVID-19 in 2020 and 2021. To date, there are no results to be found for the trial. However, as AFN reported, a whistleblower for The Remdesivir Papers came forward to expose that “across the Department of Defense, 64% of COVID patients who died between March 2020 and March 2024 were treated with remdesivir.”
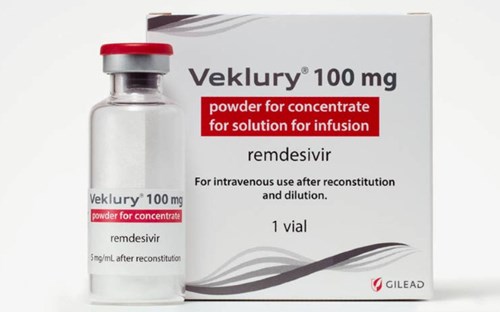 That has motivated Miller to strongly urge the incoming Trump administration to hold USAMRC accountable for not only the adverse events that followed the COVID-19 shot mandate, but also the large number of deaths associated with the use of remdesivir.
That has motivated Miller to strongly urge the incoming Trump administration to hold USAMRC accountable for not only the adverse events that followed the COVID-19 shot mandate, but also the large number of deaths associated with the use of remdesivir.
“As Americans, we want to believe that we are a nation of laws, and that we are a nation that is governed by the rule of law,” he shares. “That means that regardless of your position, your rank, your status, or your influence, you are still bound by the same laws that all other Americans are also bound by.
“If we as Americans are to hold that this is true, then there needs to be some accountability and some measure of justice implemented for those who have broken these laws.”
In an effort to gather more information from service members and veterans about clinical trials and more using remdesivir to treat COVID-19 in 2020 and 2021, the Uniformed Services Justice & Advocacy Group (USJAG) is hosting "Operation Mis-Informed Consent." Evidence suggests that informed consent forms that may have – or may not have – been provided to individuals who were treated with remdesivir were not only inaccurate, but quite deceptive.